Symmetric and Asymmetric Supercapacitor Fabrication based on Green Synthesized NiO Nanoparticles and Graphene
A. Zemieche, L. Chetibi, D. Hamana, S. Achour, V. D. Noto
Том 86 №2
370 просмотров;
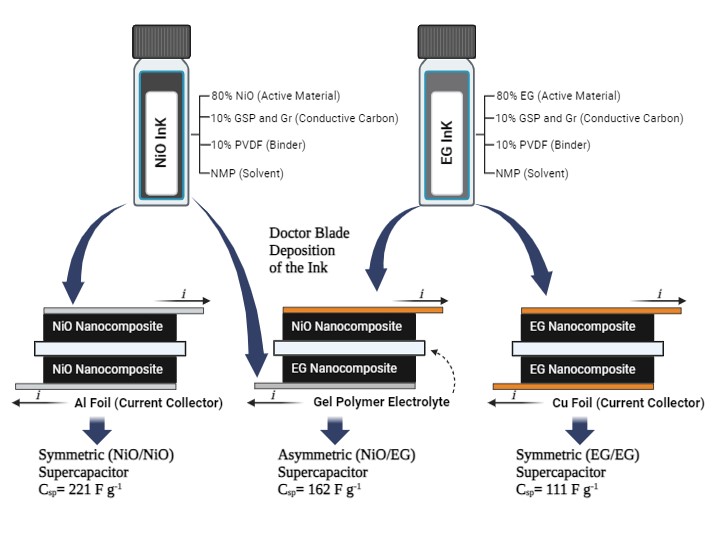
Nickel oxide nanoparticles (NiO NPs) are synthesized using olive leaf extract (OLE), which contains polyphenols. These polyphenols serve as reducing and caping agents, stabilizing the nanoparticles. Aqueous nickel acetate is employed as a precursor. Simultaneously, exfoliated graphene (EG) is obtained via electrochemical exfoliation of graphite in aqueous solutions. These materials are employed as electroactive components in supercapacitor applications. Characterization of NiO and EG involved thermogravimetric analysis (TGA), X-ray diffraction (XRD), Raman spectroscopy (RS), X-ray photoelectron spectroscopy (XPS), and scanning/transmission electron microscopy (SEM/TEM), alongside Brunauer-Emmett-Teller (BET) analysis, confirming the formation of crystalline NiO NPs with a cubic phase and Fm-3m space group. Micrographs revealed nanoscale dimensions for both NiO and EG (size) with a substantial surface area, as verified by BET analysis. Symmetric (NiO/NiO, EG/EG) and asymmetric (NiO/EG) supercapacitors are fabricated using the doctor blade method. Electrode evaluation, employing field-emission scanning microscopy FESEM, cyclic voltammetry (CV), and electrochemical impedance spectroscopy (EIS), demonstrated promising morphological and electrochemical characteristics. At low scan rates, both symmetric and asymmetric supercapacitors exhibit a notable gravimetric capacitance (221, 111, and 162 F g-1 at 1 mV s-1). Additionally, they reveal higher power density (173, 137, and 161 W kg-1 at 10 mV s-1) and show casing pseudocapacitive and electric double layer capacitor (EDLC) behavior for NiO NPs and EG, respectively.
This research significantly contributes to valuable insights by presenting a sustainable synthesis route for NiO NPs, developing high performance supercapacitor electrodes and achieving a comprehensive understanding of the electrochemical behavior of NiO NPs and EG.