Electrochemical Synthesis of Silver Nanoparticles Using Camellia chrysantha Flower Extract: Characteristics and Antibacterial Activity
H. T. Nguyen, L. M. Hoang, H. T. Nguyen, P. H. Nguyen, T. T. V. Hoa, T. T. T. Nhung, T. Q. Huy, D. C. To
Том 87 №1
377 просмотров;
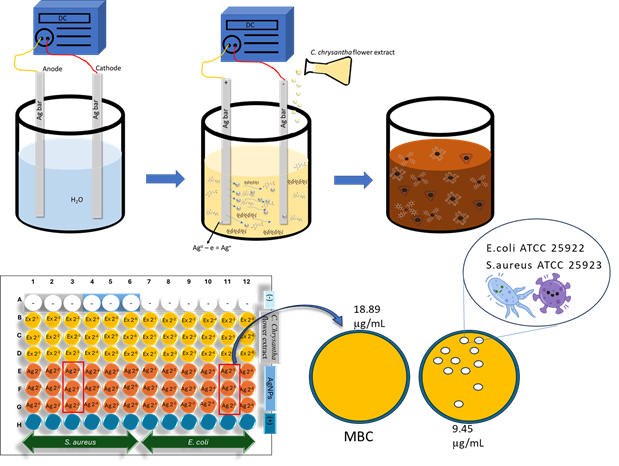
Plant extracts are powerful agents in the green synthesis of metal or metal oxide nanoparticles for biomedical applications, as they are environmentally friendly and contain no toxic chemicals. This study used the electrochemical method with the Camellia chrysantha flower extract to synthesize green silver nanoparticles (AgNPs). The extract served as an electrolyte, reducing agent, and stabilizer. Without chemicals, the synthesized nanoparticles were characterized by ultraviolet-visible spectroscopy (UV-Vis), X-ray diffraction (XRD), transmission electron microscopy (TEM), and zeta potential methods. The antibacterial activity of AgNPs against Escherichia coli and Staphylococcus aureus was evaluated using minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) methods. The results indicate successful synthesis of AgNPs with a size distribution of 4-14 nm, an average size of 8.20 nm, and spherical morphology. The AgNPs synthesized by the electrochemical method using C. chrysantha flower extract exhibited a zeta potential of -29.7 mV, indicating good dispersion, and demonstrated high antibacterial activity against both Gram-positive and Gram-negative bacterial strains. Given the traditional use of C. chrysantha flowers in food and cosmetics, the synthesis of AgNPs from this extract offers potential applications in various fields including cosmetics, food, and medicine.