Assessment of the Effects of Potassium Salts and Temperature on the Assembly Nature of Cetyltrimethylammonium bromide in the Presence of Chlorpheniramine Maleate Drug
Md. Rafikul Islam, Md. Rehan Alam, Md. Eanur Rahman, Malik Abdul Rub, Khalid A Alzahrani, and Md. Anamul Hoque
Том 88 №3
108 просмотров;
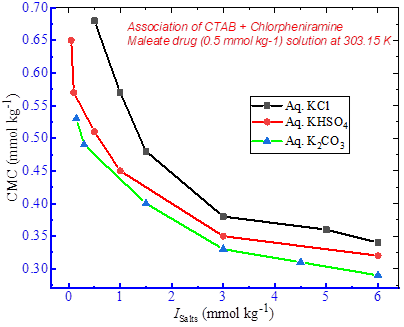
Potential interactions between cetyltrimethylammonium bromide (CTAB) and chlorpheniramine maleate (CPM) drug were explored in aqueous as well as aqueous K-salts (KCl, KHSO4, and K2CO3) media by using the conductivity measurement technique. Single critical micelle concentration (CMC) was determined during the association of CTAB/CPMmixtures in aqueous and aqueous K-salts media. The values of CMC showed an increasing trend with the rise in experimental temperatures from 298.15 K to 323.15 K. CMC of CTAB in aqueous CPM drug solution decreased compared to those observed in pure CTAB aqueous medium. The values of CMC determined in all CTAB/CPMmixtures with different K-salt media exhibited a decreasing trend in comparison to those obtained from the aqueous CTAB/CPMmixtures. Micelles formation was found to occur at lower amphiphile concentrations in the presence of various K-salts media. The standard free energy changes (∆Gmo ) were negative, suggesting the spontaneous aggregation of the components present within the CTAB/CPMmixtures. Assessment of standard enthalpy changes ( ∆Hmo ) and entropy changes (∆Smo ) revealed the electrostatic and hydrophobic interactions between CTAB and CPM species in different potassium salts media. Various other significant thermodynamic parameters, including transfer properties (∆Gm, tro , ∆Hm, tro , ∆Sm, tro ), molar heat capacity (∆Cm, po ), intrinsic enthalpy gain (∆Hmo,* ) and compensation temperature (Tc) were examined and evaluated for the CTAB/CPMmixtures in different experimental media, and the results were interpreted accordingly. These interesting results provide significant information on the surfactant-drug interactions in different potassium salts media, which will be highly useful for future research in developing the formulation procedures of various drugs and related pharmaceutical excipients.