Impact of Glucose and Urea on Micellization of the Cetyltrimethylammonium Bromide - Sulfathiazole System Under Different Temperature Conditions
Abhishek Srivastava, Ikechukwu Ugbaga Nkole, Krishna Srivastava, Neetu Srivastava
Том 88 №4
54 просмотров;
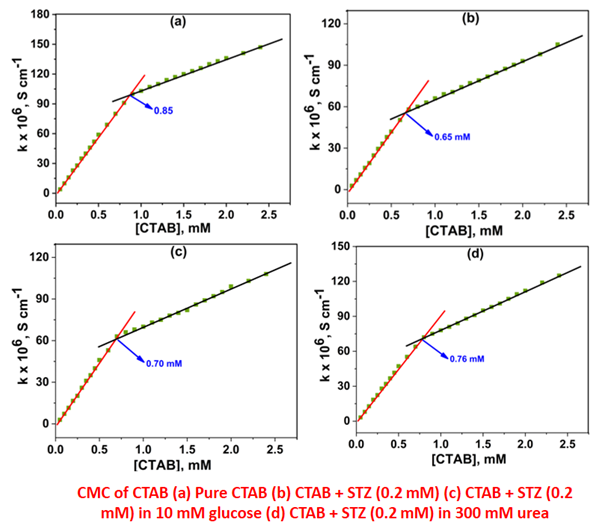
The present investigation employs a conductometric approach to investigate the influence of differing concentrations of the antibiotic drug sulfathiazole (STZ) and the additives glucose/urea on the micellization behavior of the cationic surfactant cetyltrimethylammonium bromide (CTAB) in a slightly acidic environment (рН 5.0) across a range of temperatures. The aim of this study is to elucidate the influence of surfactant-drug interactions on micelle formation and dynamics, thereby establishing the possibility of advancing drug delivery systems and enhancing the therapeutic efficacy of drugs. Unlike CTAB in pure water, the CMC (critical micellar concentration) values of CTAB decreased in the presence of STZ, i.e. micellization was facilitated. Both glucose and urea increase the CMC of the CTAB + STZ system. Unlike urea, which destabilizes water structure and diminishes hydrophobic contacts, glucose stabilizes the water structure, making it harder for CTAB molecules' hydrophobic tails to form micelles. The relationship between the CMC of the CTAB + STZ and temperature displayed a linear trend for both aqueous media and aqueous glucose/urea environment. Thermodynamic parameters (change in entropy of micellization, ΔS0m; enthalpy of micellization, ΔH0m; and Gibbs free energy of micellization, ΔG0m) and physicochemical variables (CMC and counter ion dissociation, α) have been used to characterise the interaction between CTAB and STZ. The negative ΔG0m values reveal that the CTAB + STZ mixture undergoes spontaneous micellization in both pure water and aqueous glucose/urea environments. The values of −ΔH0m and +ΔS0m for the CTAB + STZ mixture indicate that both electrostatic and hydrophobic interactions play a crucial role in aggregation.