Cilnidipine-Loaded Niosomes: A Novel Transdermal Delivery System for Enhanced Permeation
A. A. Madhav, D. D. Kambli, C. E. M. DCruz, L. Kumar, R. K. Shirodkar
Том 87 №5
189 просмотров;
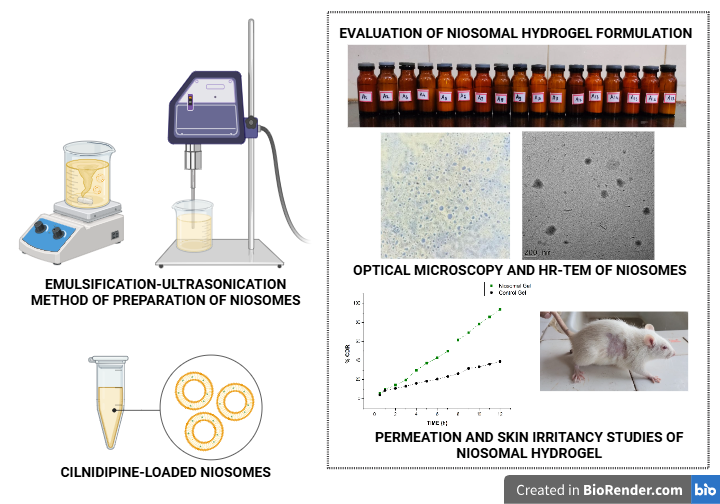
The objective of the study was to formulate Cilnidipine-loaded niosomes using cholesterol and Span 60 using the emulsification-ultrasonication method. The Box-Behnken design was adopted to optimize process variables in order to attain lower particle size and higher entrapment efficiency. Niosomal dispersion was evaluated for particle size, zeta potential, and entrapment efficiency. Morphological and drug release characteristics were also studied. The Cilnidipine niosomes were further developed as a hydrogel and evaluated for its physical appearance, viscosity, swelling characteristics, pH, spreadability, release characteristics permeation, skin irritation potential, and stability. The niosomes exhibited particle size in the range of 138.1 to 355.5 nm and drug entrapment of 82.63 to 91.77%. At the end of 8 hours, 89.06% of the drug was released. Morphological studies revealed the spherical nature of the niosomal vesicles. Fourier transform infrared spectroscopy, Differential scanning calorimetry, and X-ray diffractometry studies were also performed. Rat skin permeation studies showed a greater transdermal flux (0.4952 mg/cm².h) and permeation coefficient (0.09904) as compared to the control gel. Clove oil in cilnidipine niosomes acts as a vesicular membrane-fluidizing agent for greater drug delivery across the stratum corneum. This, along with Span 60, reduced the stratum corneum barrier rigidity, thereby enhancing its delivery across the skin. The present study thus demonstrated that Cilnidipine niosomal formulation could be a favourable transdermal delivery system for the treatment of hypertension.