Sorption of thiocyanate ions on Cr-polymer composite and obtaining a new sorbent
V. Gutsanu, V. Costis
Том 88 №1
225 просмотров;
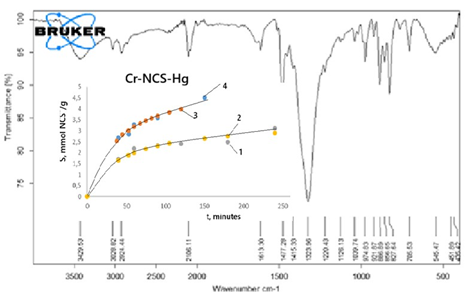
The sorption of thiocyanate ions under various conditions on a Cr-polymer composite and, for comparison, on a strongly basic anion exchanger Purolite A-400(Cl) was studied. Temperature has a significant effect on sorption on the composite and a minor effect on Purolite A-40(Cl). The sorption of thiocyanate ions on both sorbents practically does not depend on the pH of the solution in the range 2-12. The rate of thiocyanate ion sorption is limited by mixed intra-particle and boundary layer diffusion. The sorption isotherms obtained at 18 and 60 oC on Purolite A-400 are well described by the Langmuir sorption model, and on the Cr-polymer composite – by the Freundlich model. The difference in the magnitude of sorption on these composites is explained by the fact that on Purolite A-400(Cl) the retention of thiocyanate ions occurs as a result of ion exchange, while on the Cr-polymer composite it occurs as a result of ion exchange and complex formation. The Cr-polymer composite loaded with thiocyanate ions becomes a sorbent with selective sorption properties in relation to heavy metal cations, which is confirmed by the retention of Hg2+ ions from solution.